It’s Electric: Understanding—and Reducing—Static Electricity During Grinding | 25, Issue 21
Drs. JOSHUA MÉNDEZ HARPER and CHRISTOPHER H. HENDON discuss the chemistry and physics behind the electrification of coffee during grinding, its effect on extraction, and strategies to mitigate it. All images are adapted from their recent academic publication “Moisture-Controlled Triboelectrification During Coffee Grinding,” published in Matter.[1]
When I first became a barista, I was captivated by the sheer amount of information available on how to prepare coffee well. Asking “how” to do something— how to brew the most vibrant, consistent espresso, for example—turned up dozens of approaches and considerations, both in person and online. Asking “why” we use certain techniques turned up at least a handful of working theories, some based on empirical evidence and others based on anecdotal experience. While all of these (sometimes overlapping, sometimes conflicting) recommendations inspired curiosity and experimentation, I often felt overwhelmed and struggled to identify the best techniques to put into practice.
Today, whenever an innovation or breakthrough triggers a reconsideration of the knowledge we reference and skills we use across the specialty coffee industry, I find myself reflecting on similar questions. Now, though, my focus is that of a trainer and curriculum developer: Where do “best practices” come from, and what makes them “best”? Early in my coffee career, best practices seemed to be techniques that achieved some minimal threshold of mainstream adoption—or were endorsed by certain industry celebrities. Today, however, we are fortunate to have access to an increasing number of research studies that guide how we think about coffee preparation.
Take the preparation breakthroughs of the past few years: Who could forget the 2016 study from Uman et al. that demonstrated the benefits of chilling coffee before grinding?[2] Or the 2020 study from Cameron et al. that explored how to manipulate grind settings and water pressure to reduce the amount of coffee we needed to prepare espresso, all while improving beverage consistency?[3] Sometimes, research inspires new practices (I think about the flash-chilling trend that swept through the competition space after the 2016 study made headlines). In other cases, techniques that felt effective behind the bar are affirmed through the empirical backing that comes from these studies.
This illuminating piece from study authors Joshua Mendez Harper and Christopher Hendon is an example of the latter, explaining their recent research into the role of moisture in mitigating static electricity while grinding coffee. They found that adding a spritz of water to beans before grinding not only resulted in less grinder retention and cleaner workstations, but also reduced clumping in the coffee bed, decreasing the variability of extraction and resulting in more consistent, reproducible flavors in the cup. The study adds another layer of credibility to the Ross Droplet Technique, a long-discussed practice in enthusiast circles that involves stirring a very small amount of water into a dose of coffee beans before grinding.
How will this research show up in our collective barista practice moving forward? Only time will tell as professionals continue to test the technique and its operational feasibility for themselves—which, as you’ll read, the authors encourage you to do.
Chelsea Dubay
Curriculum Director
The act of grinding coffee can generate large amount of electrostatic charge, which not only profoundly affects cup quality, but speaks to some of the oldest unsolved mysteries in material science.
Around 600 BC, Thales of Miletus noted that amber, when rubbed against wool or cats’ fur, acquired a mysterious force that would attract bits of parchment paper, lint, or hay. These observations, so the story goes, constituted the discovery of the triboelectric effect—the process by which surfaces exchange and accumulate electrostatic charge when rubbed against each other (commonly referred to as “static electricity”). Although recent investigations suggest that Thales’ tale is largely doubtful,[4] triboelectricity (from the Greek τρίβω: “to rub” and ηλεκτρoν: “amber”) is certainly real and is likely the earliest manifestation of electricity known to humans.[5] Virtually any set of surfaces can charge frictionally: amber against wool, a bike tire rolling over concrete, ground coffee tumbling out of a grinder. One surface is left with positive charge; the other becomes negatively charged. Some of the effects may be mundane and familiar—the electric shock you may feel when you touch the doorknob after dragging your feet against carpeted flooring—but others are more dramatic: volcanic lightning storms[6] or the formation of planets from charged cosmic dust[7] are also a result of this kind of static electricity. Surprisingly, despite being such a well-known phenomenon, a complete understanding of why and how materials generate static remains elusive.
While physicists scratch their heads over where static comes from, baristas and other coffee professionals deal with it daily. Grinding—the action of reducing whole coffee beans into fine powders—is underpinned by repeated, disruptive frictional contacts between coffee particles and a grinding element (e.g., burrs), and these conditions invariably lead to intense charging. For example, coffee ground on a Mahlkonig EK43 grinder (Figure 1) may collect up to tens of nano-Coulombs per gram (nC/g),[8] a level of electrification comparable to that measured within severe thunderclouds or in electrified volcanic plumes (Figure 2a). Occasionally, coffee charging may be sufficiently strong to generate small sparks. More commonly, however, static charge gives rise to electrostatic forces that cause grounds to become attracted to one another and to surfaces (much like Thales’ supposed amber and paper bits). Unsurprisingly—particularly for those who spend most of their time behind an espresso machine—the finer you grind, the more prominent static electricity becomes (Figure 2b).[9] These attractive forces are detrimental in the preparation of coffee for a number of reasons. Most obviously, they manifest as clumping (“aggregation”) in the particle bed, increased grinder retention, and messy workspaces. But there are more insidious consequences for brewing: clumping changes the way the solid particles of ground coffee interface with brewing water, leading to variability in how the water will move through the coffee (“variable bed porosity”). In other words, aside from the fact that coffee seems to get everywhere and takes longer to clean, electrostatic charging may have a direct impact on our ability to replicate our brewing recipes and resulting cup quality—especially for espresso.
Figure 1. Our experimental setup to measure charge on ground coffee. For this research, all coffee was ground on a Mahlkonig EK43 grinder with stock 98 mm burrs, and we measured charge by allowing grounds to collect in a Faraday cup (a metal cup designed to catch charged particles for measurement).
Figure 2. On the left (a): Maximum charge-to-mass ratios obtained during coffee grinding compared to those reported in other particle systems like thunderclouds and volcanic ash plumes, as reported in scientific literature. On the right (b): As coffee is more finely ground, the more prominent the electrification becomes (show as “charge-to-mass ratio"). This increase in charging causes aggregation (clumping) processes become important. The inset in (b) shows a coffee clump composed of a large particle (boulder) with innumerable fines “glued” to it by electrostatic forces.
Understanding Static: Where and How?
If we wish to address electrification during grinding and its effects on brewing, we need to understand where and how the electricity is generated. The act of grinding, i.e., fragmentation and friction, certainly catalyzes charging, but the generation of static electricity is ultimately controlled by a material’s microscopic surface chemistry.[10] The complexity of material surface properties, and how environmental conditions influence these properties, is in no small way responsible for our poor understanding of static electricity in general. For example, a surface electrified at a high temperature may gain a wildly different electric charge to the charge obtained by a seemingly identical material exposed to cold. To understand this complexity specifically in coffee, we explored the charging of around 30 different commercially sourced coffees when ground (Figure 3) and observed a large scatter in the data regardless of whether we were looking for a relationship between the static generated and the roast color (Figure 3a) or the static generated and the water content of the coffee (Figure 3b). Although there was some correlation between these factors and the electric charge generated (light roasts charge positively, whereas darker ones gain negative charge), these relationships are statistically “weak.” This may be because of the compound effects of things like variability across growing, processing, and roasting conditions—all the characteristics of a green coffee and its roasting ultimately change its final chemical composition, which then influences the generation of static electricity during grinding.
To better test these relationships, we took a single green coffee—a washed Ethiopian Yirgacheffe without any defects—and developed two sets of roast profiles so that we could isolate the effects of color and residual water content on static electricity (Figure 4). The sets differed in the length of the “soak-in” period.[11] We then roasted the coffee with both of these profiles by systematically increasing the total length of the roast as well as the maximum temperature (Figure 4a). When comparing the shortest (i.e., lightest) of these profiles to the longest (i.e., darkest) of these profiles, we observed behaviors similar to those seen in commercially sourced coffees: the darker (drier) roasts charged negatively, whereas the lighter (with more residual moisture) roasts gained positive charge. Again, we found that electrification correlates weakly to color (i.e., there is a lot of “scatter” in the data), with a transition from positive to negative charging at Agtron colors of 70–80 (Figure 4b). In terms of moisture, however, we saw a stark departure from the behavior observed in commercially sourced coffees: although a transition from negative to positive charging still occurred once a coffee had a water content of approximately 2%, we found the relationship between moisture and charging to be exponential, not linear (Figure 4c). This kind of relationship has been observed in other processes, including a study on bananas,[12] and we found a much stronger correlation. This means that the residual internal moisture of a roasted coffee is an excellent predictor of the resulting electric charge, regardless of color. In other words: roast color, while providing a touchpoint for flavor, does not yield sufficient information about the chemical composition and the static electricity it generates, but internal water content does appear to be a primary factor in terms of what static is generated (and how much), making it a useful predictor.
Figure 3. Summary of electrification behaviors for approximately 30 commercial coffees as functions of (a, left) Agtron color and (b, right) residual moisture content. Charging seems to correlate weakly to roast color but slightly more to water content: the individual points in (a) don’t follow the dotted red line as much as the individual points in (b). Darker (drier) roasts tend to gain negative charge, whereas lighter roasts (which retain more moisture) charge positively. Although not shown here, charging does not seem to depend on origin or process.
Figure 4. Electrification behavior of a single coffee (a washed Ethiopian Yirgacheffe) roasted with different profiles in a systematized manner. Left (a): The longest and shortest of the roast profiles tested. Middle (b): The impact of roast color on static generated; as with the commercial samples, this graph shows a “weak” correlation between static generated and roast color. Right (c): The impact of residual moisture content and static charge; a clear, exponential relationship exists.
Managing Static: Just Add Water
Having defined the parameters that lead to charging in coffee, we now turn to strategies to deal with static electrification. One may be tempted to use more grounded metal components in the construction of grinders with the hope that some of the charge becomes funneled away (much like a grounded lightning rod serves to earth discharges during thunderstorms). A problem here is that dry coffee is an insulator, meaning it does not readily conduct electrical charge. So, while the charge on the side of a particle in direct contact with grounded metal will be neutralized, the charge on particle surfaces at any distance from these grounded components will remain in place (Figure 5a). Because of this, grounding alone may lead to more problems than solutions: even at a distance, trapped charge will be attracted to grounded surfaces, causing particles to stick to them. While grounding strips may keep particles from fluttering away, they can potentially increase retention (Figure 5b).
There are two possible solutions to the problem of trapped charge on insulating particles: either increase the mobility of the charge (by making the particles more conductive, say) or “inject” charge of the opposite polarity into the mass of ground coffee to neutralize immobile charge. The first approach to electrostatic charge reduction (increasing charge mobility) has been implemented by the coffee community (baristas in particular) for a long time. Referred to occasionally as the “Ross Droplet Technique” (RDT)[13] by baristas and enthusiasts alike, a small amount of water is added, using a spray bottle or dropper, to whole beans before grinding. This addition of free water (i.e., water not contained within the coffee) theoretically increases the conductivity of particle surfaces, activating pathways for trapped positive and negative charges to recombine or to flow toward grounded surfaces. Beyond increased recombination, added water may change the surface composition of coffee through electrochemical reactions, perhaps leading to more inefficient charging in the first place. Our next step in understanding the static electricity generated through grinding was to explore whether or not the addition of water reduced static.
Figure 5. Grounding is insufficient. (a): Schematic showing the interaction between charged particles and a metal, grounded surface. Charges in contact with the surface may be neutralized, but charges further away (even on the same grain!) may not be able to flow to ground owing to the insulating properties of coffee. These charges still exert forces that keep particles attracted to the grounded surface. (b): Photograph of charged particles coating a grounding strip on an EK43 grinder’s chute. While this strip may reduce scatter of charged particles, it does not reduce retention.
Figure 6. The impact of water content on static charge and particle size distribution. Left (a): The addition of small amounts of water significantly reduces electrification regardless of “dry” charge polarity. Right (b): This charge reduction mitigates clumping, but also drastically reduces grinder retention (inset).
To do this, we ground a subset of the same commercially sourced coffees[14] along with an increased amount of water, from 0 to 20 μL per gram of whole beans to measure changes in both the presence of static and clumping (“particle charge” and “particle distribution,” respectively, Figure 6). Even at very low water contents, we noticed a marked decrease in electrification (Figure 6a)—but at the highest content of 20 μL/g, the addition of water reduced the charge-to-mass ratio by half. Also, as charge decreased, so did the capacity for coffee particles to clump: this “deaggregation” resulted in a shift in particle size distributions toward smaller diameters, reflecting clumps breaking up into individual grains (Figure 6b). Did less static actually mean less retention in the grinder? Yes: the addition of 10 μL of water per gram decreased retention for a dark roast from over 10% to around 2.5% (inset Figure 6b).
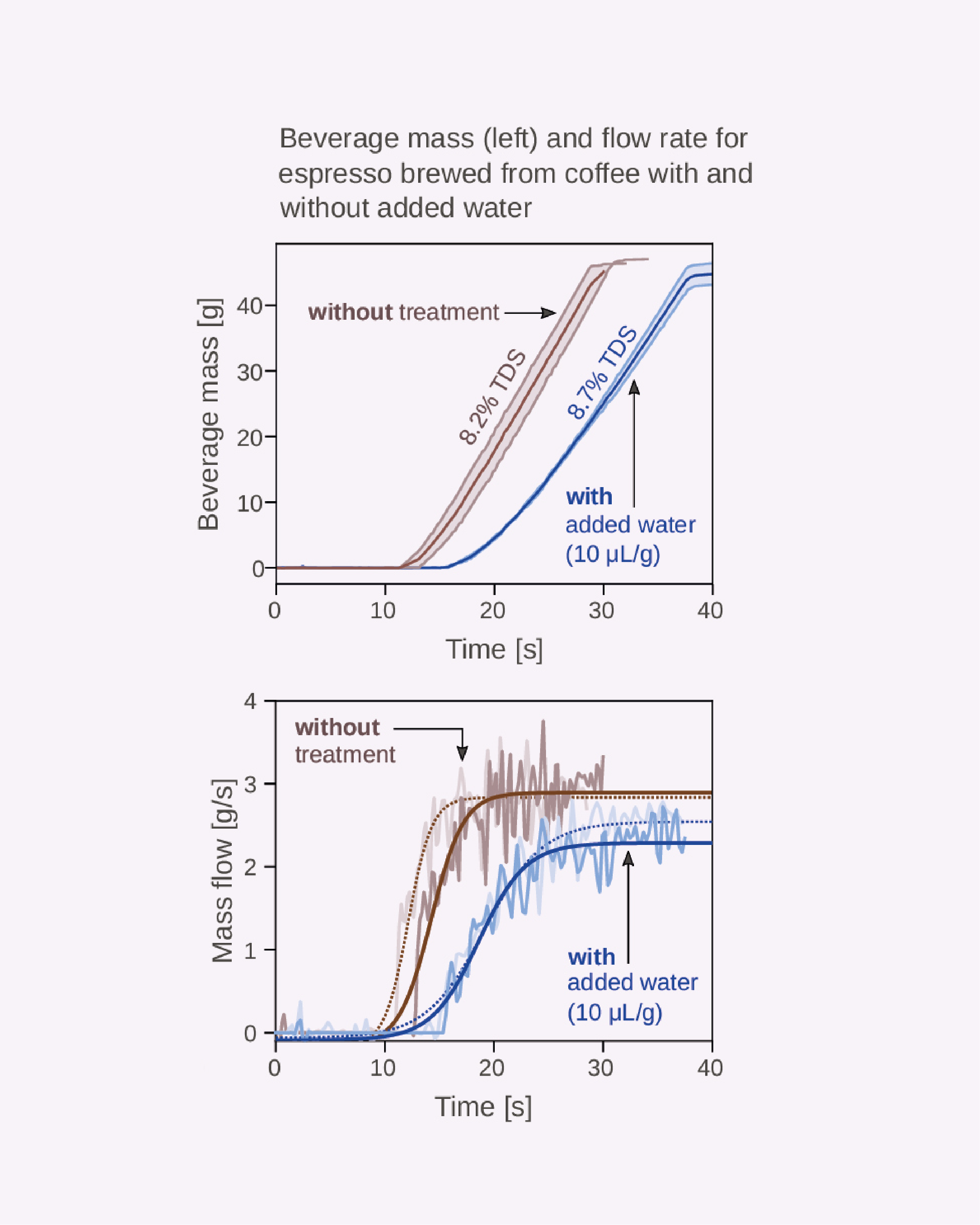
Figure 7. Impact of adding water pre-grind on the beverage mass and flow dynamics of espresso. In both graphs, the brown line shows the dynamics of espresso prepared without pre-grind water; the blue line, dynamics of espresso prepared with pre-grind water. The addition of water increases the shot time (left panel) and decreases the flow rate (right panel) and also leads to an 0.6% TDS increase.
While these effects felt a little obvious, our step was to explore what impact the addition of water during grinding had on brewing. To answer this, we prepared espressos to assess their time-dependent extraction characteristics in relation to the amount of water added to the coffee before grinding.[15] When we added 10 μL/g of water before grinding, we noticed two important things: espresso shots took nearly 50% longer to reach the desired volume out than when brewed with coffee that hadn’t received a water spray before grinding. They also demonstrated a markedly decreased flow rate (Figure 7). We noticed a change in cup concentration, too: the espresso prepared with dry ground coffee yielded 8.2% total dissolved solids (% TDS), but the espresso prepared with the “wet” coffee produced a cup with 8.7% TDS.
From a physics perspective, we believe this change in TDS is due to an increased bed density in the portafilter, as the fines and boulders would not be electrostatically attracted to one another. This means that, as clumps fall apart, fines are able to efficiently “pack” the bed, allowing water to more uniformly contact coffee during the shot. In their 2020 paper, Cameron and others[16] suggest that a finer grind may yield uneven and variable extraction (due to uncontrolled bed porosity), but our findings suggest that the addition of water to roasted coffee before grinding may provide a direct remedy to this problem by homogenizing the bed.
The increase in concentration afforded by added water (~10–15%) should certainly affect taste, but also has important cost-saving implications for the coffee industry, which is worth US$343.2 billion or 1.5% of the US gross domestic product. And these experiments have only highlighted the changes in TDS and flow rates for a single grind size! There’s more to learn about how RDT impacts coffee preparation using other brew ratios and grind settings. If you are comfortable with adding small amounts of water to your grinder, we hope this inspires you to explore these variables yourself.
The fact that so many coffee professionals are familiar with the idea of adding a squirt of water to coffee before grinding, together with the data presented here, suggests that the practice resolves problems involving clumping, channeling, and poor extractions. However, as too much water may promote caking or corrosion within the grinder, there has been recent interest in developing “dry” electrostatic dissipation techniques. These methods generally involve the production of free ions—that is, negatively or positively charged gas molecules that neutralize charges on coffee grains. We investigated this approach in another study, and found that its effectiveness depends greatly on the coffee being ground (namely, roast color and moisture content) and at what point the ions are added (e.g., at the output of the grinder or within the grinding cavity itself).[17] This variance stands in contrast to the Ross Droplet Technique, which reduces the static charge regardless of coffee color or residual water content (see Figure 6). Beyond the application of water and ionization, there may be other charge-reduction strategies, like selecting burr materials with anti-static coatings or designing grinders with charge-dissipative geometries (like the static wicks on the wings of aircraft); exploring these approaches is something worth doing in detail.
So far, electrostatic charging during coffee grinding has been cast in a rather negative light, but there may be some benefit to be gained from this pervasive electrification. Perhaps this charging behavior reveals chemical and physical qualities unseen by other metrics; after all, as we have said above, contact and frictional electrification arise from these very material properties. In fact, such ideas have been pursued vigorously in other fields: volcanologists, for example, have spent the last 20 years linking particle charging to the amount of ash, steam, and other compounds erupting from active volcanoes.[18] Other researchers have shown that the static caused by certain plastics is sensitive to contaminants in liquids and gasses, allowing for the detection of harmful chemicals in the environment.[19] Could similar methods be used to pinpoint defects in whole beans or monitor the composition and homogeneity of blends? The possibilities of static as a diagnostic tool are exciting but virtually unexplored in the context of coffee.
While the application of electrochemistry to coffee may seem like a fairly niche—albeit exciting!—area of study, the insights we have gained from our experiments extend well beyond the cup, impacting our knowledge of electrification processes associated with pharmaceutical powders, volcanic eruptions, and even the sand dunes of Saturn’s moon Titan.[20] Understanding charging during coffee grinding not only aids in the pursuit of the tastiest espresso, it also brings us closer to resolving long-standing questions in material science, engineering, and geophysics. ◇
Assistant Professor JOSHUA MÉNDEZ HARPER is an electrical engineer at Portland State University, conducting experimental research on obscure problems in electrostatics. Associate Professor CHRISTOPHER H. HENDON is a computational material chemist at the University of Oregon, where he runs the Hendon Materials Simulation group.
Both would like to thank students ROBIN BUMBAUGH, ELENA COPE, LEIF LINDBERG, JUSTIN PHAM, CONNOR MCDONALD, ELIAS RHEINGOLD, and LENA WEHN for their invaluable contributions to this project. Additionally, we would like to acknowledge our colleagues JOSEF DUFEK and YONG-HYUN KIM.
This work was supported by the Coffee Science Foundation, with financial underwriting from Nuova Simonelli. Read the full academic paper: https://doi.org/10.1016/j.matt.2023.11.005.
References
[1] Joshua Mendez Harper, Connor S. McDonald, Elias J. Rheingold, Lena C. Wehn, Robin E. Bumbaugh, Elana J. Cope, Leif E. Lindberg, Justin Pham, Yong-Hyun Kim, Joseph Dufek, and Christopher H. Hendon, “Moisture Controlled Fracto- and Triboelectrification During Coffee Grinding,” Matter (2023): https://doi.org/10.1016/j.matt.2023.11.005.
[2] Uman et al., “The Effect of Bean Origin and Temperature on Grinding Roasted Coffee,” Scientific Reports 6 (2016), https://www.nature.com/articles/srep24483.
[3] Cameron et al., “Systematically Improving Espresso: Insights from Mathematical Modeling and Experiment,” Matter 2, no. 3 (2020), https://doi.org/10.1016/j.matt.2019.12.019.
[4] Paul Iversen and Daniel J. Lacks, “A Life of Its Own: The Tenuous Connection between Thales of Miletus and the Study of Electrostatic Charging,” J. Electrost. 70, no. 3 (2012): 309–311, https://doi.org/10.1016/j.elstat.2012.03.002.
[5] Daniel J. Lacks and R. Mohan Sankaran, “Contact
Electrification of Insulating Materials,” J. Phys. Appl. Phys. 44, no. 45 (2011): 453001, https://doi.org/10.1088/0022 3727/44/45/453001.
[6] Corrado Cimarelli, Sonja Behnke, Kimberly Genareau, Joshua Mendez Harper, and Alexa R. Van Eaton, “Volcanic Electrification: Recent Advances and Future Perspectives,” Bull. Volcanol. 84, no. 8 (2022): 78, https://doi.org/10.1007/s00445-022-01591-3.
[7] Tobias Steinpilz, Kolja Joeris, Felix Jungmann, Dietrich Wolf, Lothar Brendel, Jens Teiser, Troy Shinbrot, and Gerhard Wurm, “Electrical Charging Overcomes the Bouncing Barrier in Planet Formation,” Nat. Phys. 16, no. 2 (2020): 225–229, https://doi.org/10.1038/s41567-019-0728-9.
[8] To maintain consistency with other works, we report electrification as charge-to-mass (Q/m) ratios with units of nano-Coulombs per gram. A nano-Coulomb is equal to the charge on approximately 6.24 billion electrons.
[9] Firstly, per gram, smaller particles have more extensive surface areas than larger particles. As the surface area involved in frictional interactions increases, so does the potential for triboelectrification. Further, as coffee breaks into smaller and smaller bits, fragments gain additional charge through fracto-electrification—charging
arising from the emission of ions and electrons during the formation of new surfaces. Lastly, fines are lighter than boulders, meaning that electrostatic forces rather than gravitational ones tend to dominate their behavior (that is, fines are more likely to adhere to surfaces than larger fragments).
[10] Daniel J. Lacks et al.: 453001.
[11] There are many ways to manage the internal temperature of green coffee that take both the latent heat of a roasting drum as well as the heat of the burners into account. “Soaking” is a technique where a roaster cuts the ignition of the burners once green coffee has been added to the drum (“charged”) for a period of time before reigniting the burners, changing the rate at which the green coffee’s internal temperature rises.
[12] Rak Dandamrongrak, Gordon Young, and Richard Mason, “Evaluation of Various Pre-Treatments for the Dehydration of Banana and Selection of Suitable Drying Models,” J. Food Eng. 55, no. 2 (2002): 139–146, https://doi.org/10.1016/S0260-8774(02)00028-6.
[13] Although the name “Ross Droplet Technique” first begins to appear on internet forums around 2004, baristas have anecdotally been dropping water in hoppers for quite a long time.
[14] Coffees were ground using the same parameters: 2.0 on the EK43.
[15] For the purposes of this study, each espresso was prepared to the following recipe: 18.0 g in, ground at 1.0 on an EK43, 45.0 g out. Additionally, each shot was tamped at 196 N of force (about the force exerted by a 20 kg weight) and brewed using 94°C water with a 2 second pre-infusion at 7 bar static water pressure.
[16] Michael I. Cameron, Dechen Morisco, Daniel Hofstetter, Erol Uman, Justin Wilkinson, Zachary C. Kennedy, Sean A. Fontenot, William T. Lee, Christopher H. Hendon, and Jamie M. Foster, “Systematically Improving Espresso: Insights from Mathematical Modeling and Experiment,” Matter 2, no. 3 (2020): 631–648, https://doi.org/10.1016/j.matt.2019.12.019.
[17] Joshua Mendez Harper and Christopher H. Hendon, “Strategies to Mitigate Electrostatic Charging during Coffee Grinding,” arXiv (2024).
[18] Corrado Cimarelli et al.: 78.
[19] Austin Chang, Cameron Uy, Xiao Xiao, Xiao Xiao, and Jun Chen, “Self-Powered Environmental Monitoring via a Triboelectric Nanogenerator,” Nano Energy 98 (2022): 107282, https://doi.org/10.1016/j.nanoen.2022.107282
[20] J. S. Mendez Harper, G. D. McDonald, J. Dufek, M. J. Malaska, D. M. Burr, A. G. Hayes, J. McAdams, and J. J. Wray, “Electrification of Sand on Titan and Its Influence on Sediment Transport,” Nat. Geosci. 10, no. 4 (2017): 260–265, https://doi.org/10.1038/ngeo2921.
We hope you are as excited as we are about the release of 25, Issue 21. This issue of 25 is made possible with the contributions of specialty coffee businesses who support the activities of the Specialty Coffee Association through its underwriting and sponsorship programs. Learn more about our underwriters here.